

Myeloid cells play a crucial role in the innate immunity and reprogram the adaptive immunity with tremendous therapeutic potential. We are leveraging our novel Myeloid Engager Platform to achieve this goal.
Role of myeloid cells in connecting innate to adaptive immunity
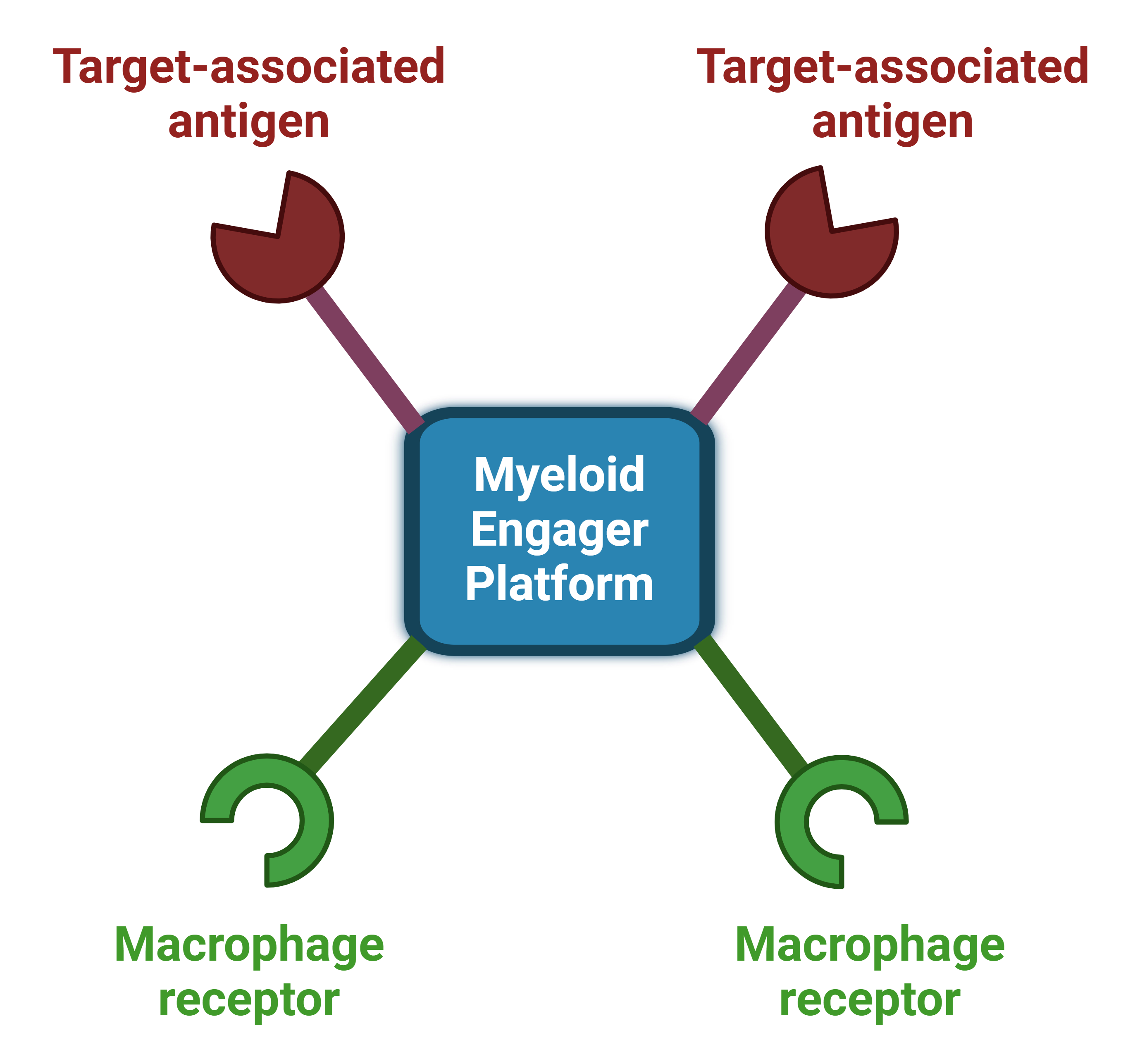
A proprietary, versatile, fully humanized, potentially first-in-class bispecific antibody platform.
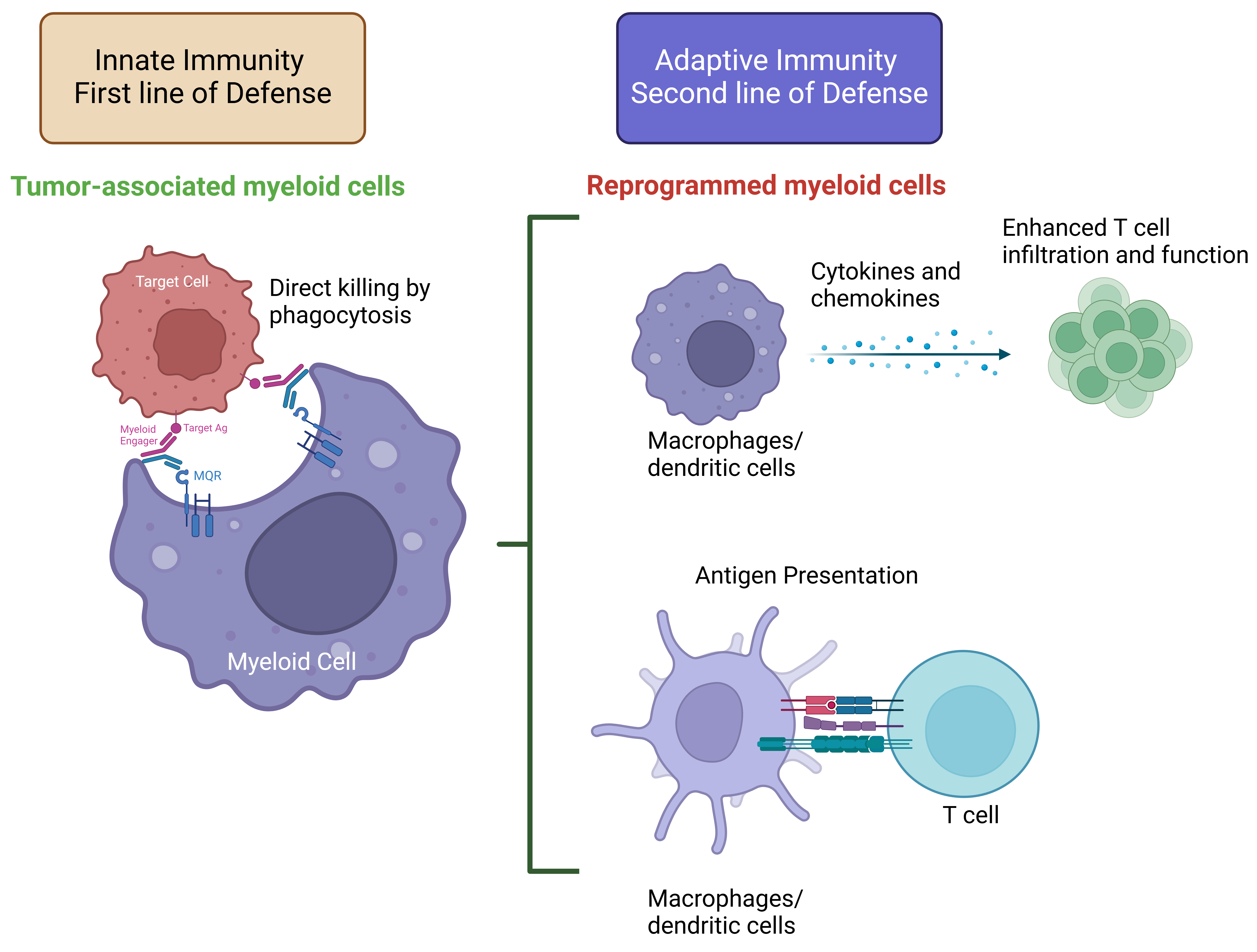
In the presence of the target cells, our platform activates myeloid cells to cause phagocytosis with minimal proinflammatory cytokine release. Thus, ensuring safety while effectively depleting targeting cells.
A universal platform that causes potent phagocytic killing of distinct target cells expressing different TAAs.
Antibody Fc engineered to enhance Fcy receptor function resulting in dual activation of both myeloid and NK cells.
Antibody engineered to increase stability, antibody titer and reduce structural heterogeneity. Thus, optimized to enhance CMC properties and developability.
Activation of phagocytosis, and ADCC (NK cells) which enables potent killing of cancer cells.
Reprogramming of myeloid cells to M1-like macrophage phenotype and activation of dendritic cells which will enhance phagocytic and antigen presentation functions and increase in pro-inflammatory cytokine release.
Phagocytosis of cancer cells by dendritic cells and macrophages resulting in tumor-specific neoantigen presentation to T cells.
Controlled and local proinflammatory cytokine release by myeloid cells enables recruitment and activation of T, NK, and myeloid cells, enhancement in phagocytosis, ADCC, and antigen presentation functions.
Furthermore, such a controlled and local cytokine release ensures safety while effectively activating myeloid cells.

为了更好的呈现效果,移动端请竖屏浏览

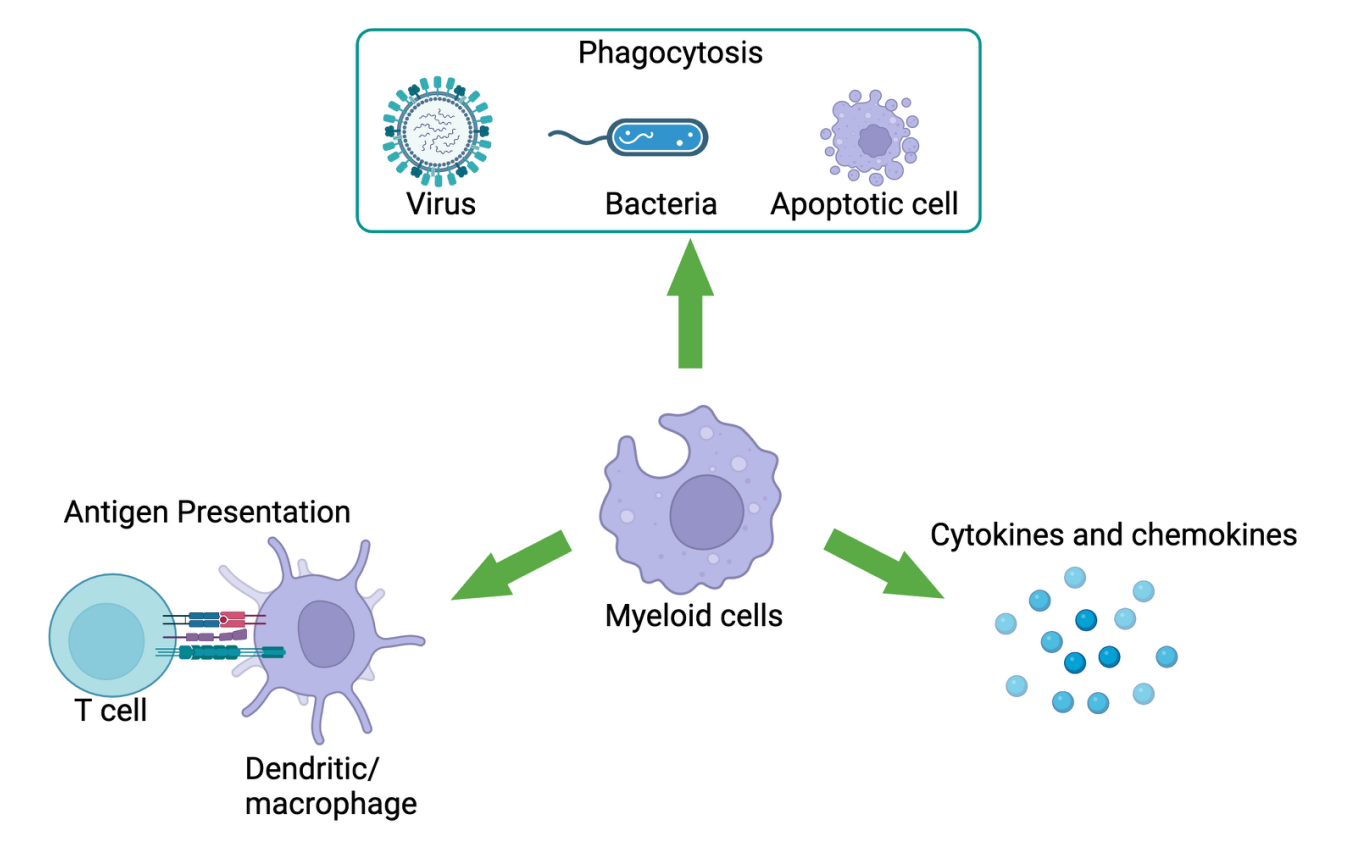
The phagocytic function of macrophages plays important roles in development, homeostasis, tissue repair, and bridging innate and adaptive immunity. In the context of homeostasis, macrophages utilize distinct receptors to engulf and eliminate different targets, such as pathogens and apoptotic cells. It is estimated that adults turn over 200–300 billion cells each day, and the majority of these are cleared by macrophages, highlighting the high capacity of phagocytosis process. In addition to clearance of targets, phagocytic signaling through innate receptors can lead to antigen presentation to T cells and the release of proinflammatory cytokines and chemokines, and these can lead to increase in immune cell infiltration and function.
Tumor-associated myeloid cells (TAMCs), comprised of macrophages (TAMs), monocytes, dendritic cells, and granulocytes (like neutrophils), are a major component of the tumor microenvironment of many solid and hematological cancers. TAMCs are known to secrete growth factors, cytokines, and chemokines that collectively promote immunosuppression, tumor growth and metastasis as well as resistance to treatments such as immune checkpoint inhibitors and chemotherapies. Thus, pharmacologically activating and directing TAMCs against cancer is a novel approach that may benefit patients. Overall, myeloid cells are considered potential therapeutic targets for cancer therapy and treatment strategies.
LTZ's Myeloid Engager cross-links, activates novel innate receptors on myeloid cells, and has therapeutic potential such as reducing tumor growth, enhancing T cell function, inducing immunological memory, and eliciting durable responses.