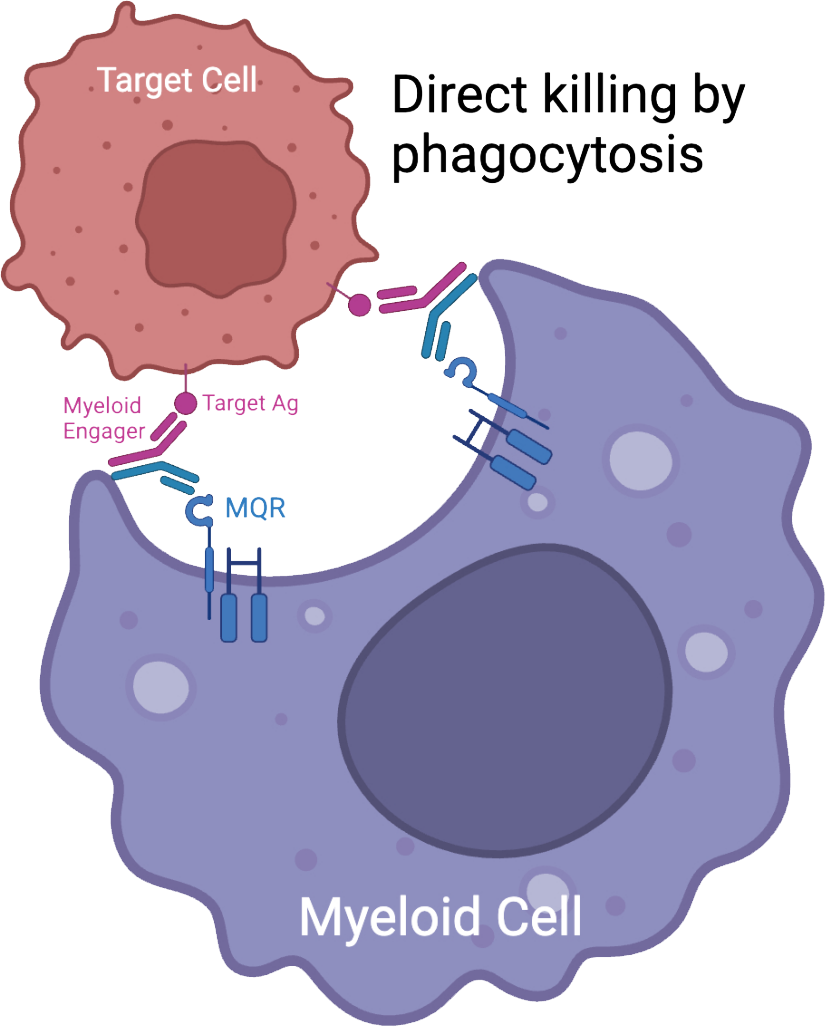
Empowering Myeloid Cells to Better Address Unmet Clinical Needs
To maximize the drugability potential, we target approaches to improve clinical benefits based on reverse translational science and emerging biology of the tumor microenvironment.
-
(Reverse)Translational Science
Clinical Experience Basic Research
Identification of patients' unmet needs, novel targets, pathways, and mechanisms.
-
Drug Discovery & Development
Myeloid Engager
Validation of target, mechanism of action, and proof of concept, and development of novel engager with high potency and good safety profile.
-
Immunotherapy
Clinical Benefit
Boosting of anti-tumor immunity to fight against cancer.